Drug Screening and Druggability of the Interactome
|
|
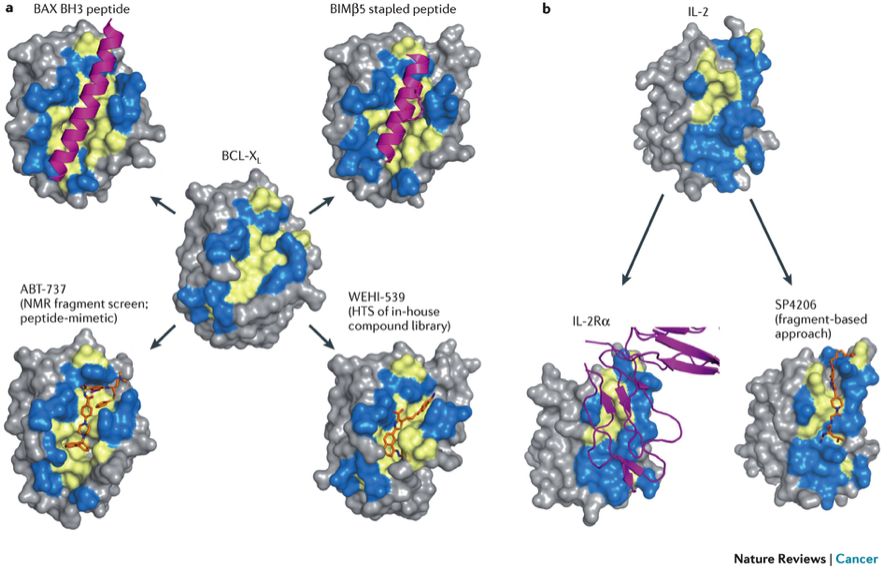
Despite their biological importance, protein-protein interactions (PPIs) have mostly been considered as poor drug targets to address human disease. However, several recent successes strongly suggest that PPIs might be more amenable to modulation than initially thought (Fig. 1; LabTube) (Arkin et al, Chem Biol, 2014; Milroy et al, Chem Rev, 2014; Nero et al, Nat Rev Cancer, 2014; Scott et al, Nat Rev Drug Discov, 2016). Today, PPIs are considered as the next generation of therapeutic targets, and most pharmaceutical industries have now extended their drug discovery programs to PPIs (Mullard, Nat Rev Drug Discov, 2012).
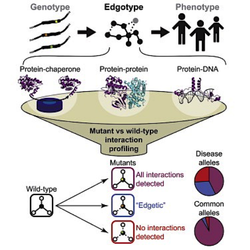
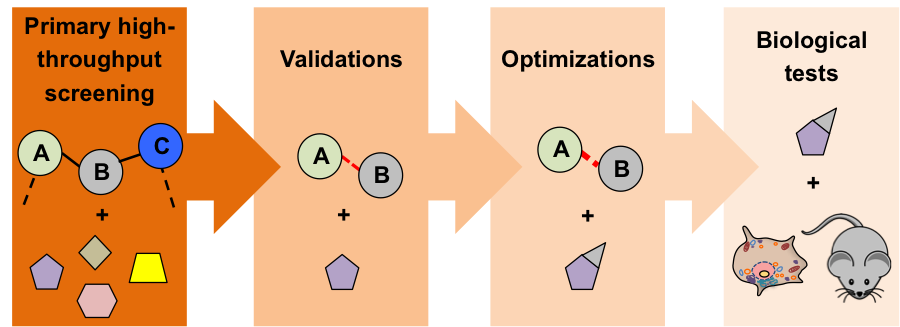
Since the full network of human PPIs is expected to contain hundreds of thousand interactions (Stumpf et al, Proc Natl Acad Sci USA, 2008), even a relatively small fraction of disruptable PPIs could vastly exceed the number of enzymes, such as kinases, conventionally chosen for drug screening. In addition, recent studies (see section Edge profiling of disease alleles) suggest that disrupting PPIs (“edges”) could have more impact in disease development than disrupting proteins (“nodes”) themselves (Fig. 2) (Zhong et al, Mol Syst Biol, 2009; Sahni et al, Cell, 2015). This clearly highlights the importance of finding modulators of PPIs to develop new therapeutics that effectively treat human diseases. Currently, less than 0.01 % of the human protein interactome (Stumpf et al, Proc Natl Acad Sci USA, 2008) has been targeted by chemical compounds (Thompson et al, ACS Chem Biol,), and two drugs targeting PPIs have already been approved by the FDA (Tirofiban and Lifitegrast) (1). This suggests that the vast protein-protein interactome remains largely unexplored for the discovery of new drugs. In this research project, we are developing new high-throughput platforms for the systematic discovery and validation of modulators of protein-protein interactions (new “chemical edgetic” approach). Our pipelines involve the use of phenotypic screens in the yeast S. cerevisiae (chemical genetic approach), as well as in cellulo and in vitro assays such as the Reverse Yeast Two-Hybrid (RY2H) (Vidal et al, Proc Natl Acad Sci USA; Vidal & Endoh, Trends Biotechnol, 1999), the NanoLuc Two-Hybrid (N2H) (Dixon et al, ACS Chem Biol, 2015) and the DNA-barcoded protein (Gu et al, Nature, 2014) technologies (Fig. 3). To help us in this effort, we are collaborating with several research groups in the US (Caroline Shamu’s group; John Beutler’s group; Liangcai Gu’s group), in France (Yves Jacob’s group; Xavier Morelli’s group) and in Belgium (Jan Tavernier’s group). Please contact Tong Hao and Michael Calderwood with your questions and comments. |